Introduction
Research in the Deming lab is focused on
synthesis, processing, characterization and evaluation of biomimetic materials
based on polypeptides. These materials are being studied since they can be
prepared from renewable resources, can be biocompatible and biodegradable, and
possess unique self-assembling properties. The Deming lab develops new
synthetic materials with properties that rival the complexity found in
biological systems. Our emphasis is on development of new synthetic
methodologies as well as the use of biological precedents and strategies for
the design of new materials. Our lab continues to take on significant new challenges
in the exploration of applications of our materials for interaction with
biological systems and for medicine, as well as development of new economical
and scalable preparative routes to more complex and functional polypeptide
architectures.
The
building blocks of synthetic polypeptides. NCAs can often be prepared
in large scale (grams to kilograms) in a single step from amino acids.
Cheng, J.; Deming, T. J. Top. Curr. Chem., 2012, 310, 1 – 26.
Cheng, J.; Deming, T. J. Top. Curr. Chem., 2012, 310, 1 – 26.
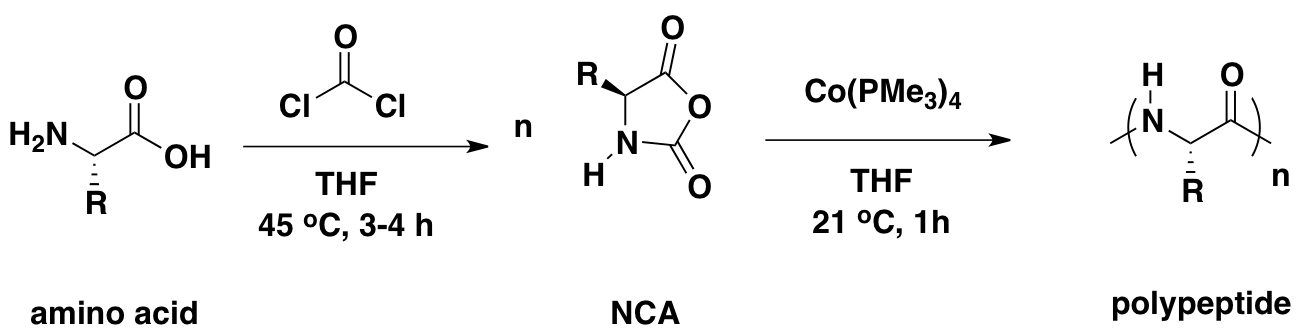
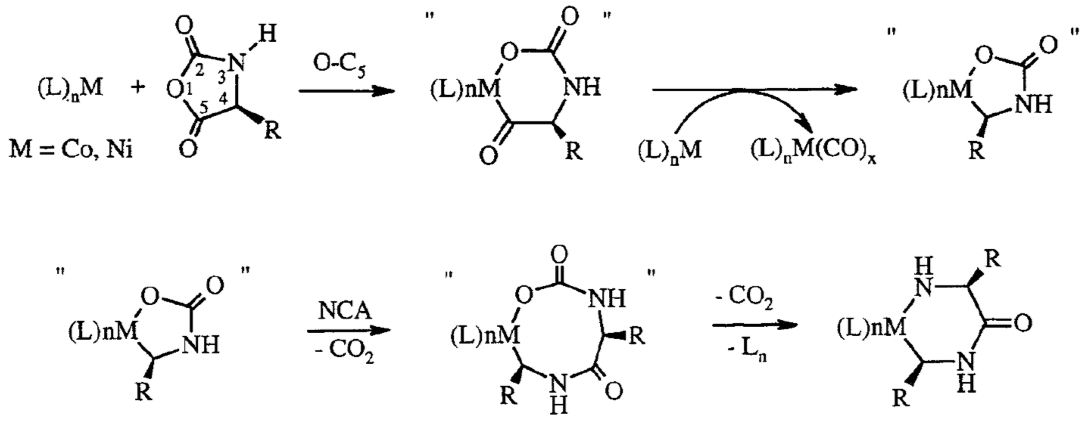
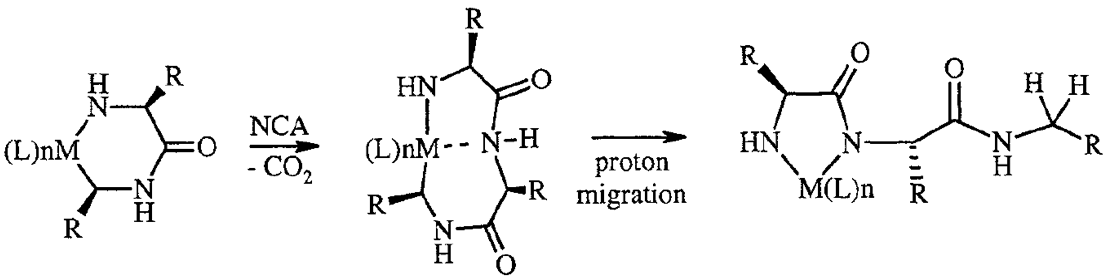
Polypeptide Synthesis via Catalysis
Our
catalysis chemistry allows allows living polymerization of NCAs with
significant rate enhancement over conventional initiators.
Deming, T. J.; Curtin, S. A. J. Am. Chem. Soc., 2000, 122, 5710-5717.
Curtin, S. A. and Deming, T. J. J. Am. Chem. Soc., 1999, 121, 7427-7428.
Deming, T. J. Macromolecules, 1999, 32, 4500-4502.
Deming, T. J. J. Am. Chem. Soc., 1998, 120, 4240-4241.
Deming, T. J. Nature, 1997, 390, 386-389.
Deming, T. J.; Curtin, S. A. J. Am. Chem. Soc., 2000, 122, 5710-5717.
Curtin, S. A. and Deming, T. J. J. Am. Chem. Soc., 1999, 121, 7427-7428.
Deming, T. J. Macromolecules, 1999, 32, 4500-4502.
Deming, T. J. J. Am. Chem. Soc., 1998, 120, 4240-4241.
Deming, T. J. Nature, 1997, 390, 386-389.
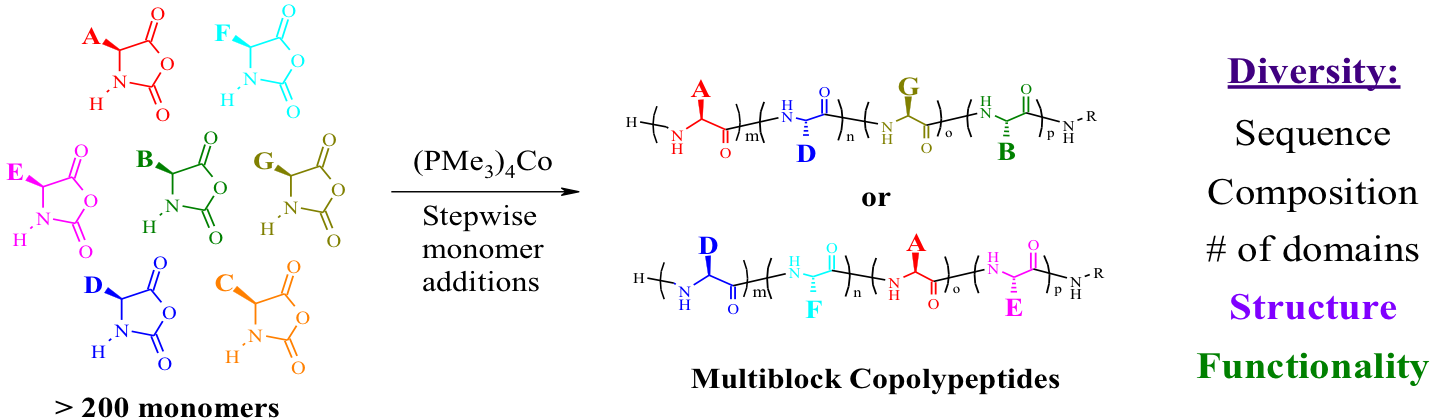
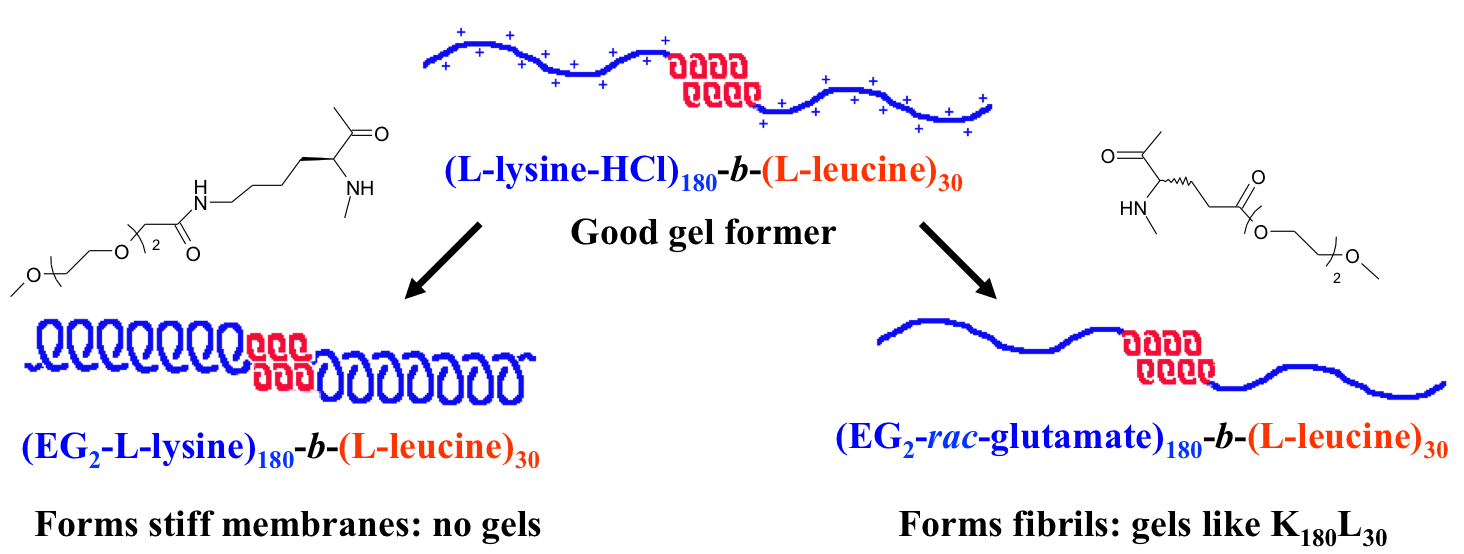
Multidomain Block Copolypeptide Self Assembly
Deming, T. J. WIREs Nanomed. Nanobiotechnol., 2014, 6, 283-297.
Hanson, J. A.; Chang, C. B.; Graves, S. M.; Li, Z.; Mason, T. G.; Deming, T. J. Nature, 2008, 455, 85-89.
Holowka, E. P.; Sun, V. Z.; Kamei, D. T.; Deming, T. J. Nature Materials, 2007, 6, 52–57.
Bellomo, E.; Wyrsta, M. D.; Pakstis, L.; Pochan, D. J.; Deming, T. J. Nature Materials, 2004, 3, 244-248.
Nowak, A. P.; Breedveld, V.; Pakstis, L.; Ozbas, B.; Pine, D. J.;Pochan, D.; Deming, T. J. Nature, 2002, 417, 424-428.
Cha, J. N.; Stucky, G. D.; Morse, D. E.; Deming, T. J. Nature, 2000, 403, 289-292.
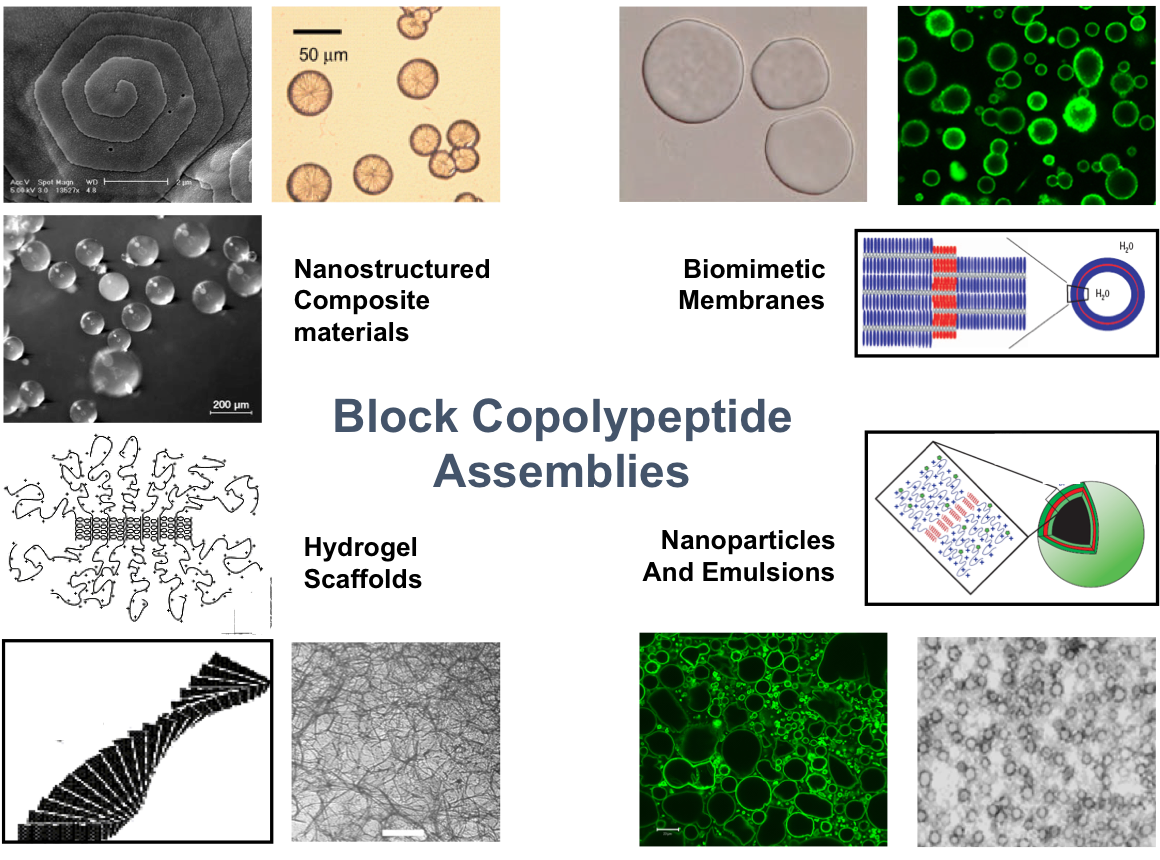
Introducing Biological Functionality into Polypeptides
Synthesis of Functionalized NCAs
The
use of functionalized NCAs allows direct incorporation of biologically
relevant functional groups in precise locations in chains, but may
require multiple synthetic steps and tedious purification.
Perlin, P.; Gharakhanian, E. G.; Deming, T. J. Chem. Commun., 2018, 54, 6196 - 6199.
Deming, T. J. Chem. Rev. 2016, 116, 786–808.
Yakovlev, I.; Deming, T. J. J. Amer. Chem. Soc., 2015, 137, 4078-4081.
Yakovlev, I.; Deming, T. J. ACS Macro Lett., 2014, 3, 378-381.
Rhodes, A. J.; Deming, T. J. ACS Macro Lett., 2013, 2, 351-354.
Rhodes, A. J.; Deming, T. J. J. Amer. Chem. Soc., 2012, 134, 19463-19467.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2012, 134, 4112-4115.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2010, 132, 15068–15071.
Kramer, J. R.; Deming, T. J. Biomacromolecules, 2010, 11, 3668 - 3672.
Perlin, P.; Gharakhanian, E. G.; Deming, T. J. Chem. Commun., 2018, 54, 6196 - 6199.
Deming, T. J. Chem. Rev. 2016, 116, 786–808.
Yakovlev, I.; Deming, T. J. J. Amer. Chem. Soc., 2015, 137, 4078-4081.
Yakovlev, I.; Deming, T. J. ACS Macro Lett., 2014, 3, 378-381.
Rhodes, A. J.; Deming, T. J. ACS Macro Lett., 2013, 2, 351-354.
Rhodes, A. J.; Deming, T. J. J. Amer. Chem. Soc., 2012, 134, 19463-19467.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2012, 134, 4112-4115.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2010, 132, 15068–15071.
Kramer, J. R.; Deming, T. J. Biomacromolecules, 2010, 11, 3668 - 3672.
PEGylated NCAs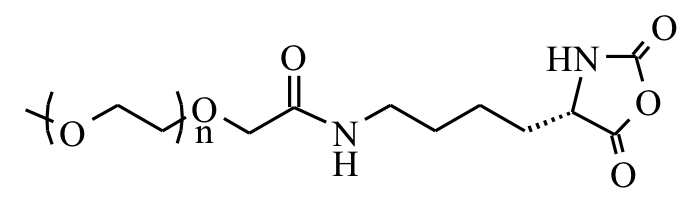 |
Glycosylated NCAs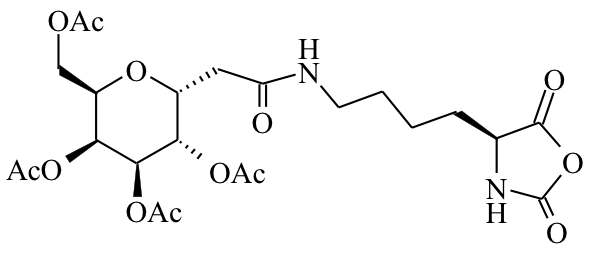 |
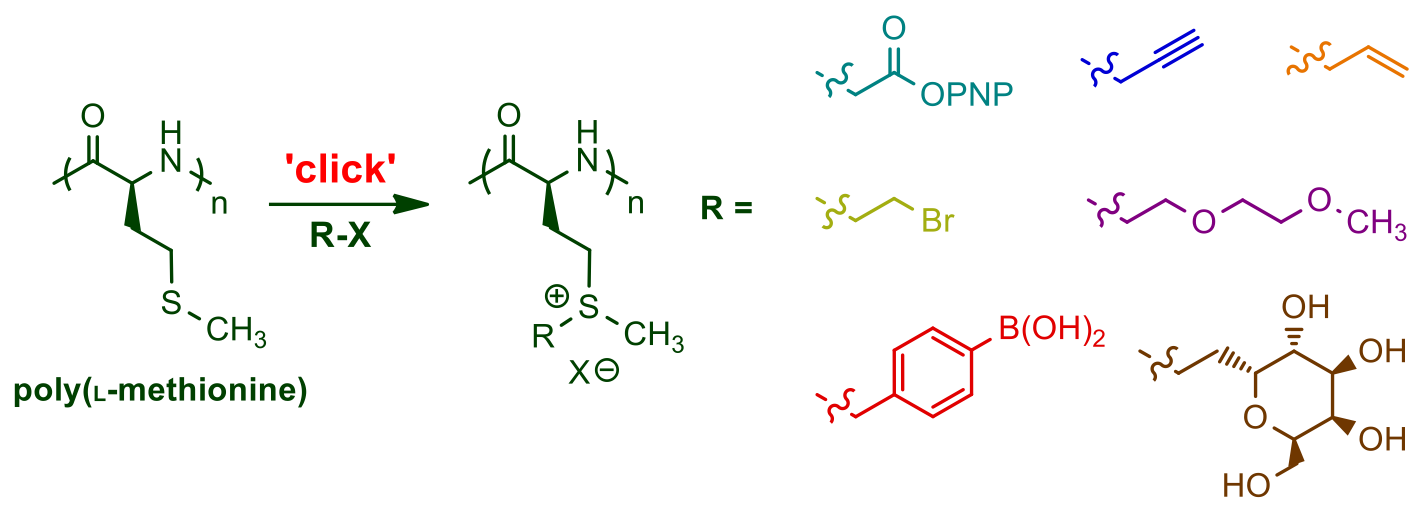
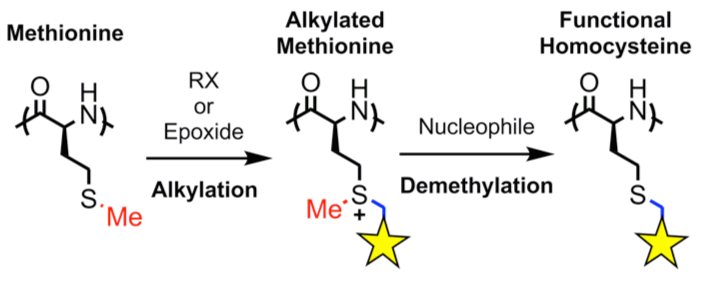
Our
methodology utilizes methionine NCA, derived from the natural amino
acid and readily prepared and polymerized without need of protecting
groups. High yield, efficient modification of thioether groups after
polymer formation provides a scalable route to highly functional, and
biologically relevant polypeptides.
Deming, T. J. Bioconjugate Chem., 2017, 28, 691−700.
Petitdemange, R.; Garanger, E.; Bataille, L.; Dieryck, W.; Bathany, K.; Garbay, B.; Deming, T. J.; Lecommandoux, S. Biomacromolecules, 2017, 18, 544-550.
Gharakhanian, E. G.; Deming, T. J. Chem. Commun., 2016, 52, 5336-5339.
Kramer, J. R.; Petitdemange, R.; Bataille, L.; Bathany, K.; Wirotius, A.-L.; Garbay, B.; Deming, T. J.; Garanger, E.; Lecommandoux, S. ACS Macro Lett., 2015, 4, 1283-1286.
Gharakhanian, E. G.; Deming, T. J. Biomacromolecules, 2015, 16, 1802-1806.
Rodriguez, A. R.; Kramer, J. R.; Deming, T. J. Biomacromolecules, 2013, 14, 3610-3614.
Kramer, J. R.; Deming, T. J. Chem. Commun., 2013, 49, 5144 - 5146.
Kramer, J. R.; Deming, T. J. Biomacromolecules, 2012, 13, 1719-1723.
Deming, T. J. Bioconjugate Chem., 2017, 28, 691−700.
Petitdemange, R.; Garanger, E.; Bataille, L.; Dieryck, W.; Bathany, K.; Garbay, B.; Deming, T. J.; Lecommandoux, S. Biomacromolecules, 2017, 18, 544-550.
Gharakhanian, E. G.; Deming, T. J. Chem. Commun., 2016, 52, 5336-5339.
Kramer, J. R.; Petitdemange, R.; Bataille, L.; Bathany, K.; Wirotius, A.-L.; Garbay, B.; Deming, T. J.; Garanger, E.; Lecommandoux, S. ACS Macro Lett., 2015, 4, 1283-1286.
Gharakhanian, E. G.; Deming, T. J. Biomacromolecules, 2015, 16, 1802-1806.
Rodriguez, A. R.; Kramer, J. R.; Deming, T. J. Biomacromolecules, 2013, 14, 3610-3614.
Kramer, J. R.; Deming, T. J. Chem. Commun., 2013, 49, 5144 - 5146.
Kramer, J. R.; Deming, T. J. Biomacromolecules, 2012, 13, 1719-1723.
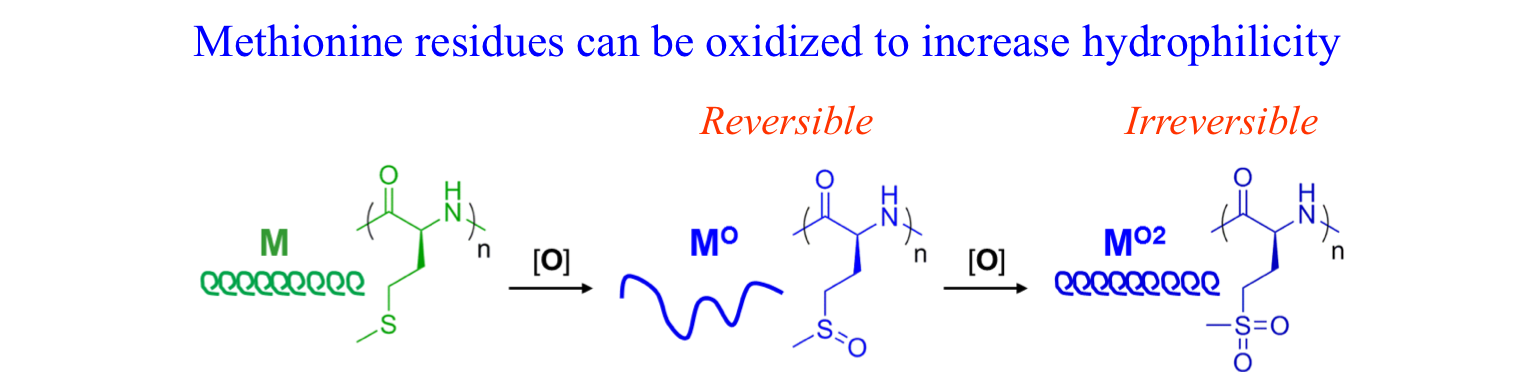
Methionine Oxidation
Methionine Alkylation and Demethylation
 |
 |
Polypeptides with Switchable Chain Conformations
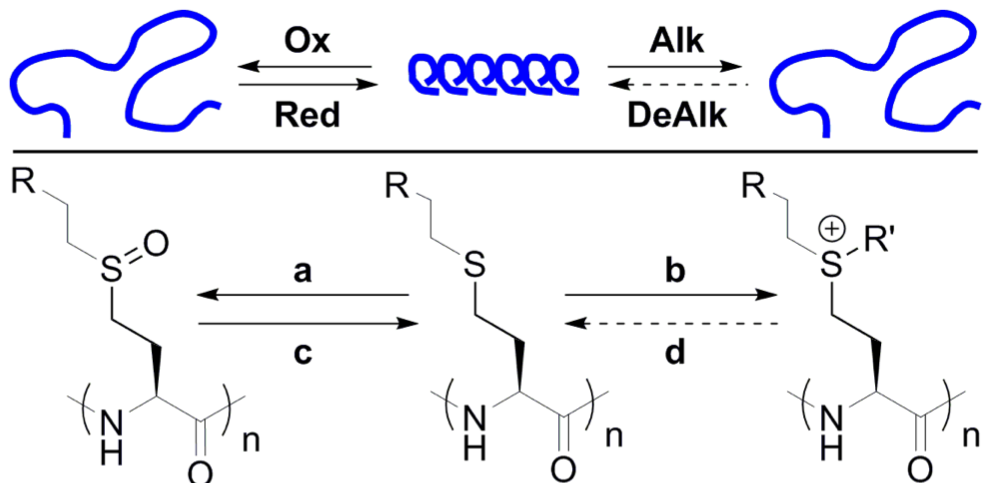
Our
lab has discovered that functional polypeptides based on
poly(homocysteine) backbones can undergo reversible switching between alpha-helical
and disordered conformations via mild, and reversible chemical
modifications under biologically relevant conditions.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2014, 136, 5547–5550.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2012, 134, 4112-4115.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2014, 136, 5547–5550.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2012, 134, 4112-4115.
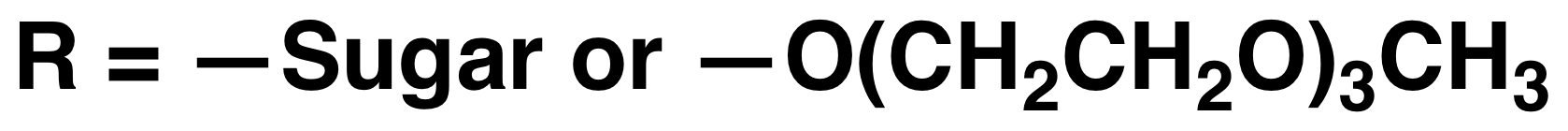
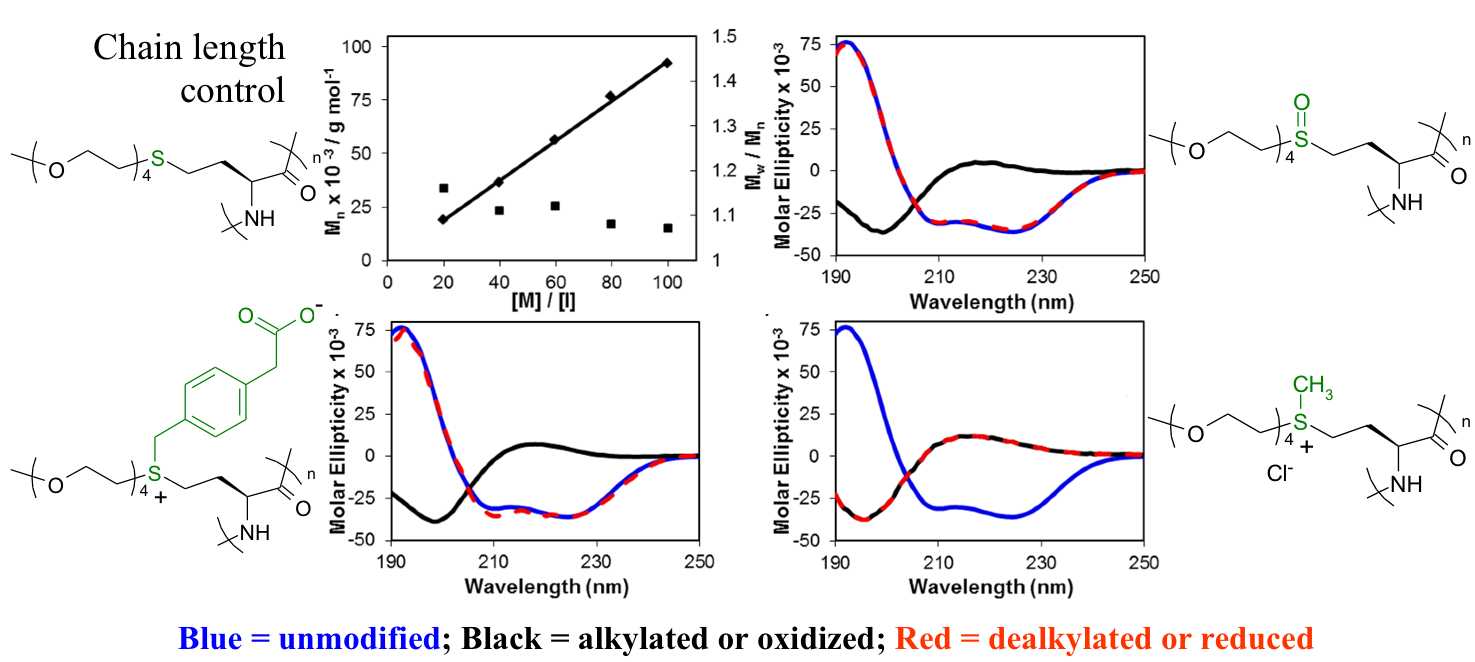
Thermoresponsive Polypeptides
Using
methionine alkylation and demethylation methodology, we prepared a
series of oligoethylene glycol functionalized poly(homocysteine)
derivatives, all from the same parent poly(methionine) sample. These
polymers undergo reversible temperature dependent solubility
transitions (lower critical solution temperature behavior) in aqueous
media. These and related polypeptides are being developed for use in
stimuli responsive block copolypeptide assemblies.
Gharakhanian, E. G.; Deming, T. J. J. Phys. Chem. B, 2016, 120, 6096-6101.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2014, 136, 5547–5550.
Gharakhanian, E. G.; Deming, T. J. J. Phys. Chem. B, 2016, 120, 6096-6101.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2014, 136, 5547–5550.
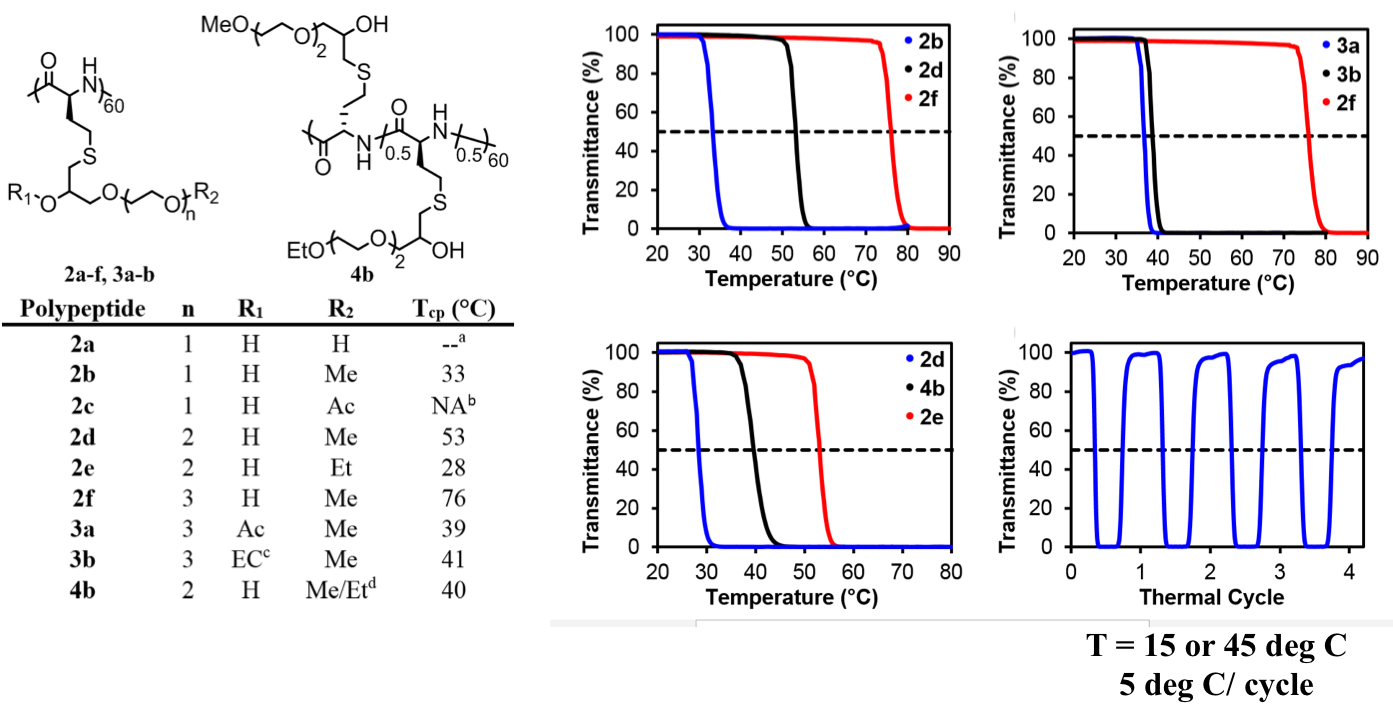
Diblock Copolypeptide Hydrogels (DCH) for Central Nervous System Repair In
collaboration with the lab of Prof. Michael Sofroniew (Neurobiology
Dept, UCLA), were are developing polypeptide hydrogels for study
of biology and neural repair in central nervous system (CNS)
tissues. Synthetic DCH have been designed with tunable physical
properties (stiffness, porosity, chemical functionality), and can be
degraded in vivo. We have
shown that many DCH formulations form hydrogel deposits and are well
tolerated in healthy mouse forebrain tissue. DCH are being developed to
encapsulate and release both hydrophilic (e.g. protein) and hydrophobic
(e.g. small molecule active agent) cargos within CNS tissues. We are
also developing DCH formulations to encapsulate neural progenitor stem
cells (NPSCs) to allow cell grafting within CNS tissues, and to provide
biomimetic scaffolds for encapsulated cells. Recent efforts have
focused on study and development of DCH formulations to facilitate
neural repair after spinal cord injury.
Anderson, M. A.; O’Shea, T. M.; Burda, J. E.; Ao, Y.; Barlatey, S. L.; Bernstein, A. M.; Kim, J. H.; James, N. D.; Rogers, A.; Kato, B.; Wollenberg, A. L.; Kawaguchi, R.; Coppola, G.; Wang, C.; Deming, T. J.; He, Z.; Courtine, G.; Sofroniew, M. V. Nature, 2018, 561, 369-400.
Anderson, M. A.; Burda, J. E.; Ren, Y.; Ao, Y.; O’Shea, T. M.; Kawaguchi, R.; Coppola, G.; Khakh, B. S.; Deming, T. J.; Sofroniew, M. V. Nature, 2016, 532, 195-200.
Yang, C-Y.; Song, B.; Ao, Y.; Nowak, A. P.; Abelowitz, R. B.; Korsak, R. A.; Havton, L. A.; Deming, T. J.; Sofroniew, M. V. Biomaterials, 2009, 30, 2881-2898.
Anderson, M. A.; O’Shea, T. M.; Burda, J. E.; Ao, Y.; Barlatey, S. L.; Bernstein, A. M.; Kim, J. H.; James, N. D.; Rogers, A.; Kato, B.; Wollenberg, A. L.; Kawaguchi, R.; Coppola, G.; Wang, C.; Deming, T. J.; He, Z.; Courtine, G.; Sofroniew, M. V. Nature, 2018, 561, 369-400.
Anderson, M. A.; Burda, J. E.; Ren, Y.; Ao, Y.; O’Shea, T. M.; Kawaguchi, R.; Coppola, G.; Khakh, B. S.; Deming, T. J.; Sofroniew, M. V. Nature, 2016, 532, 195-200.
Yang, C-Y.; Song, B.; Ao, Y.; Nowak, A. P.; Abelowitz, R. B.; Korsak, R. A.; Havton, L. A.; Deming, T. J.; Sofroniew, M. V. Biomaterials, 2009, 30, 2881-2898.
 |
 |
Encapsulation of Hydrophobic Molecules within DCH
The
hydrophobic domains of DCH are able to dissolve small hydrophobic
molecules. These can be small molecule drugs or signalling molecules.
Zhang, S.; Anderson, M. A.; Ao, Y.; Khakh, B. S.;
Deming, T. J.; Sofroniew, M. V. Biomaterials, 2014, 35, 1989-2000.
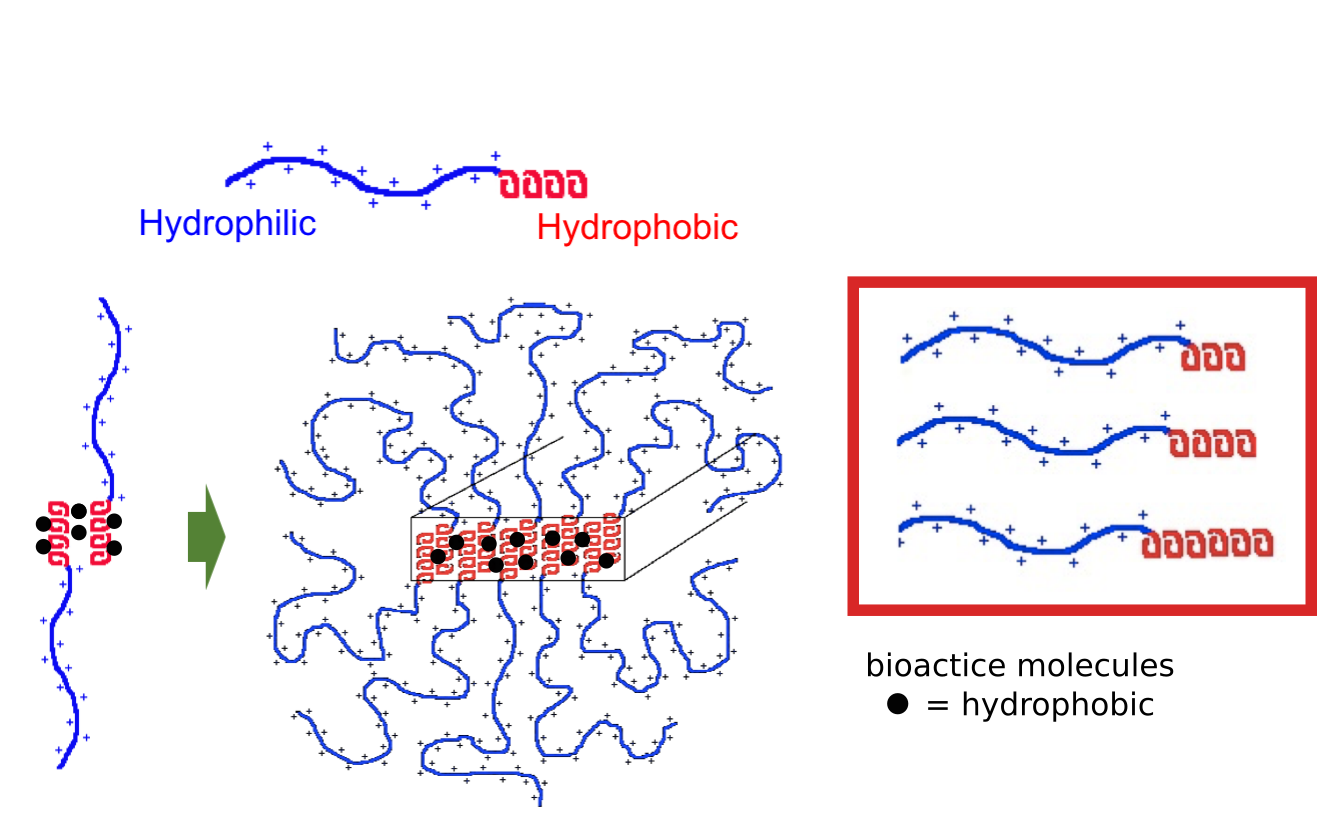
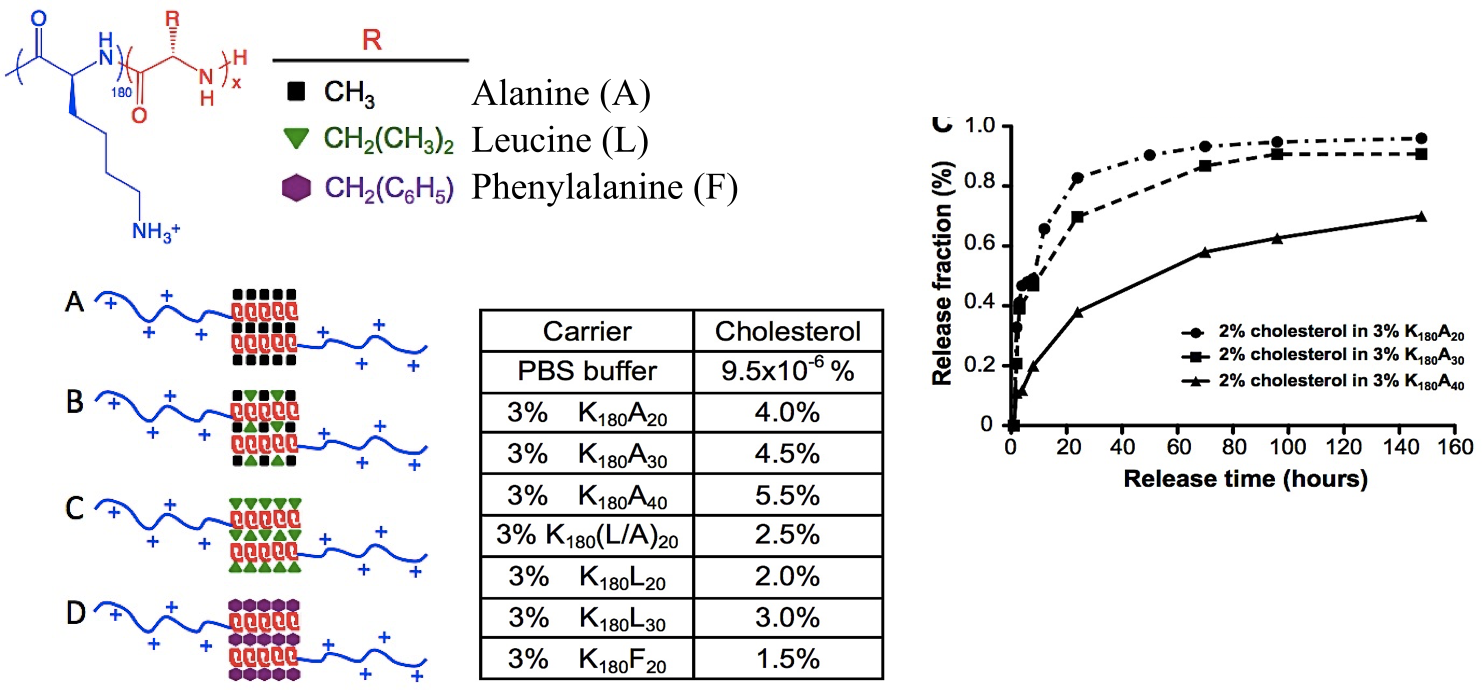
Tuning of Cargo Loading Capacity and Release Rate
Variation of hydrophobic segments in DCH allows adjustment of loading capacity and release rate
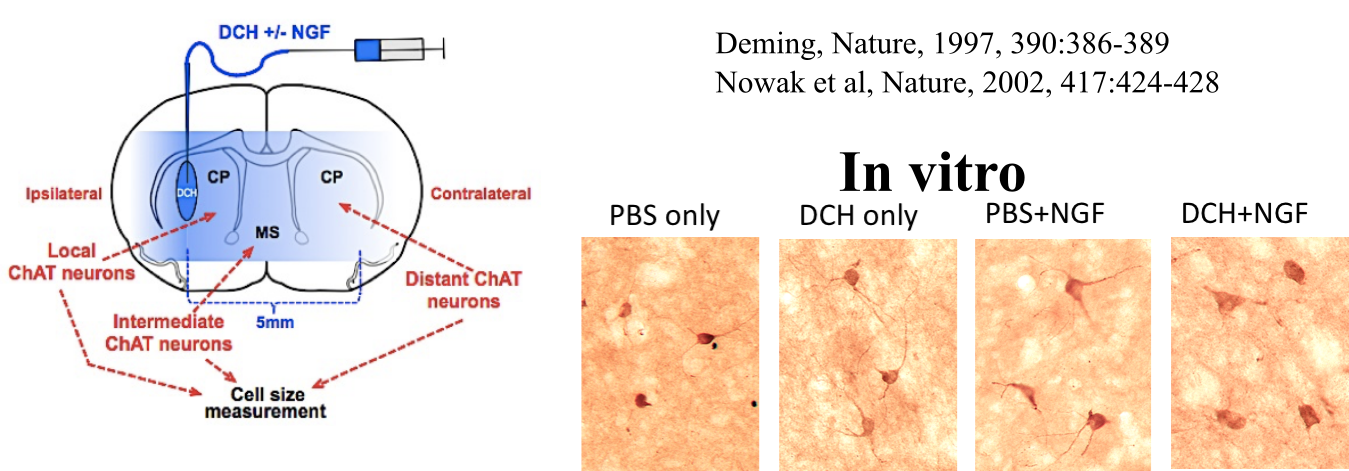
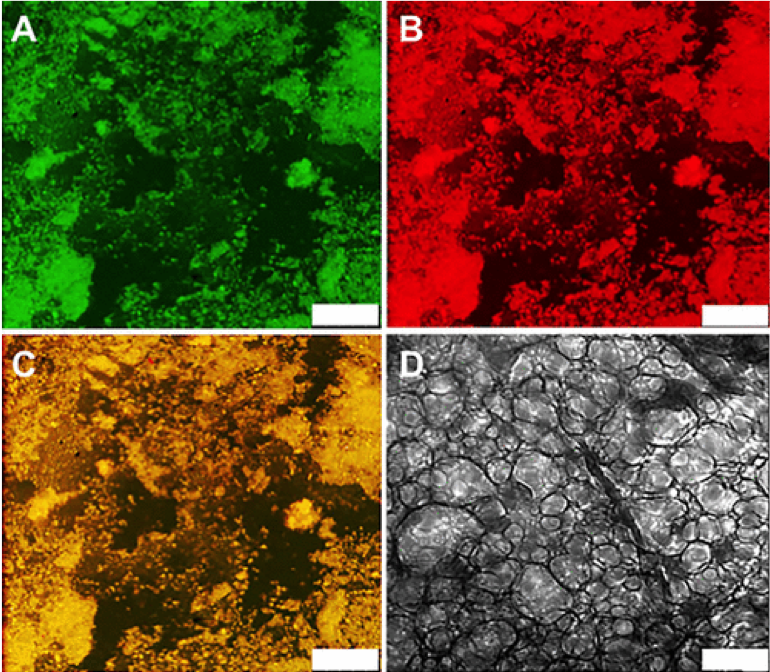
Encapsulation and Release of Growth Factors (proteins) in DCH
Release
of nerve growth factor (NGF) from a DCH depot in healthy mouse
forebrain shows biological effects on cholinergic neurons (ChAT) in vivo, where they respond to NGF by increasing in size. DCH provide prolonged release of NGF compared to injection of NGF in saline.
Song, B.; Song, J.; Zhang, S.; Anderson, M. A.; Ao, Y.; Yang, C-Y.; Deming, T. J.; Sofroniew, M. V. Biomaterials, 2012, 33, 9105-9116.
Song, B.; Song, J.; Zhang, S.; Anderson, M. A.; Ao, Y.; Yang, C-Y.; Deming, T. J.; Sofroniew, M. V. Biomaterials, 2012, 33, 9105-9116.
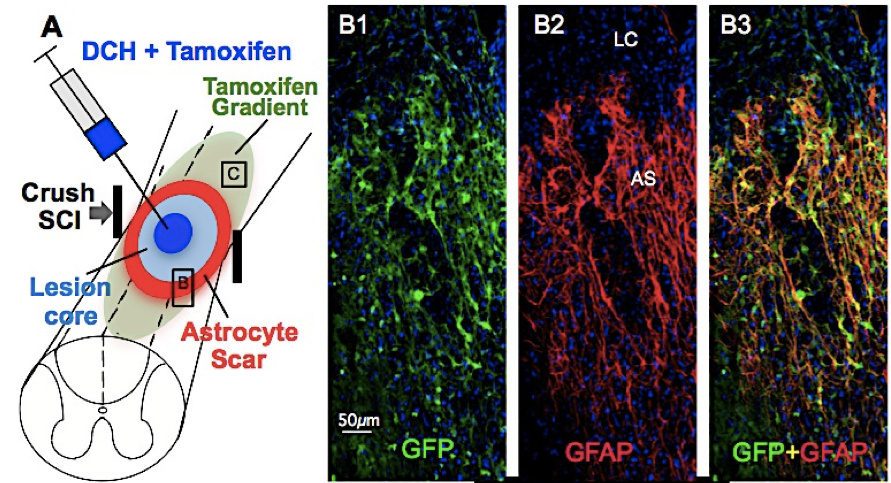
Encapsulation and Release of Small Molecules in DCH
Release
of Tamoxifen from a DCH depot in the lesion core of a spinal cord
injury model in mouse
shows biological effects on scar forming astrocytes. In the astrocytes
(also identified using GFAP), Tamoxifen activates reporter gene
expression of green fluorescent protein (GFP) in transgenic mice.
Zhang, S.; Anderson, M. A.; Ao, Y.; Khakh, B. S.; Deming, T. J.; Sofroniew, M. V. Biomaterials, 2014, 35, 1989-2000.
Zhang, S.; Anderson, M. A.; Ao, Y.; Khakh, B. S.; Deming, T. J.; Sofroniew, M. V. Biomaterials, 2014, 35, 1989-2000.
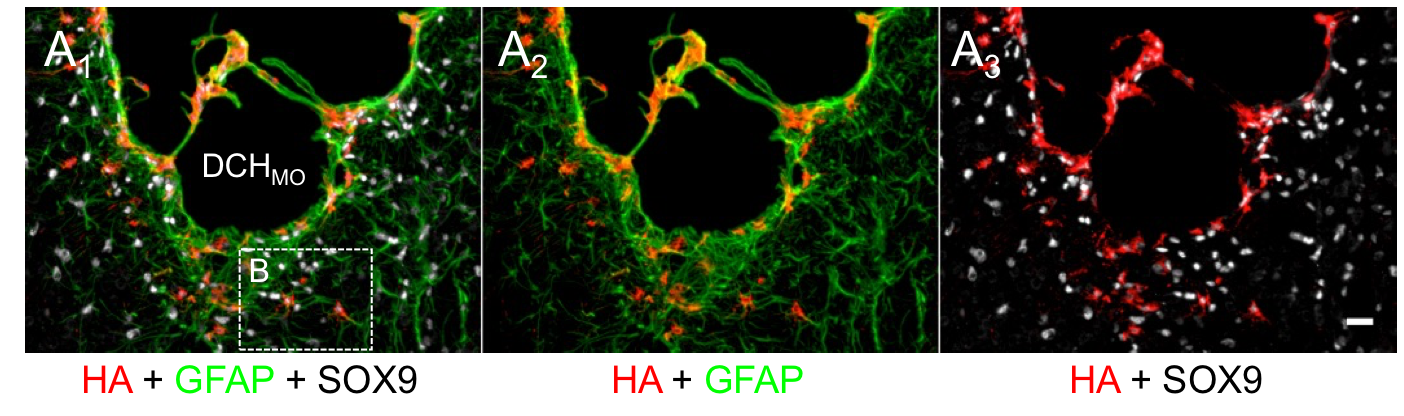
Non-Ionic DCH for Cell Grafting
To
improve cell compatibility, non-ionic DCH were developed. In the
initial design, oligoethylene glycol functionalized polypeptide
segments were used to replace cationic lysine segments found in
original DCH. These DCH were able to support encapsulated NPSCs, which
could then be grafted into CNS tissue with high viability and
integration with host tissue.
Zhang, S.; Alvarez, D. J.; Sofroniew, M. V.; Deming, T. J. Biomacromolecules, 2015, 16, 1331-1340.
Anderson, M. A.; Zhao, Z.; Ao, Y.; Cheng, Y.; Sun, Y.; Deming, T. J.; Sofroniew, M. V. ACS Biomater. Sci. Eng., 2015, 1, 705-717.
Zhang, S.; Alvarez, D. J.; Sofroniew, M. V.; Deming, T. J. Biomacromolecules, 2015, 16, 1331-1340.
Anderson, M. A.; Zhao, Z.; Ao, Y.; Cheng, Y.; Sun, Y.; Deming, T. J.; Sofroniew, M. V. ACS Biomater. Sci. Eng., 2015, 1, 705-717.
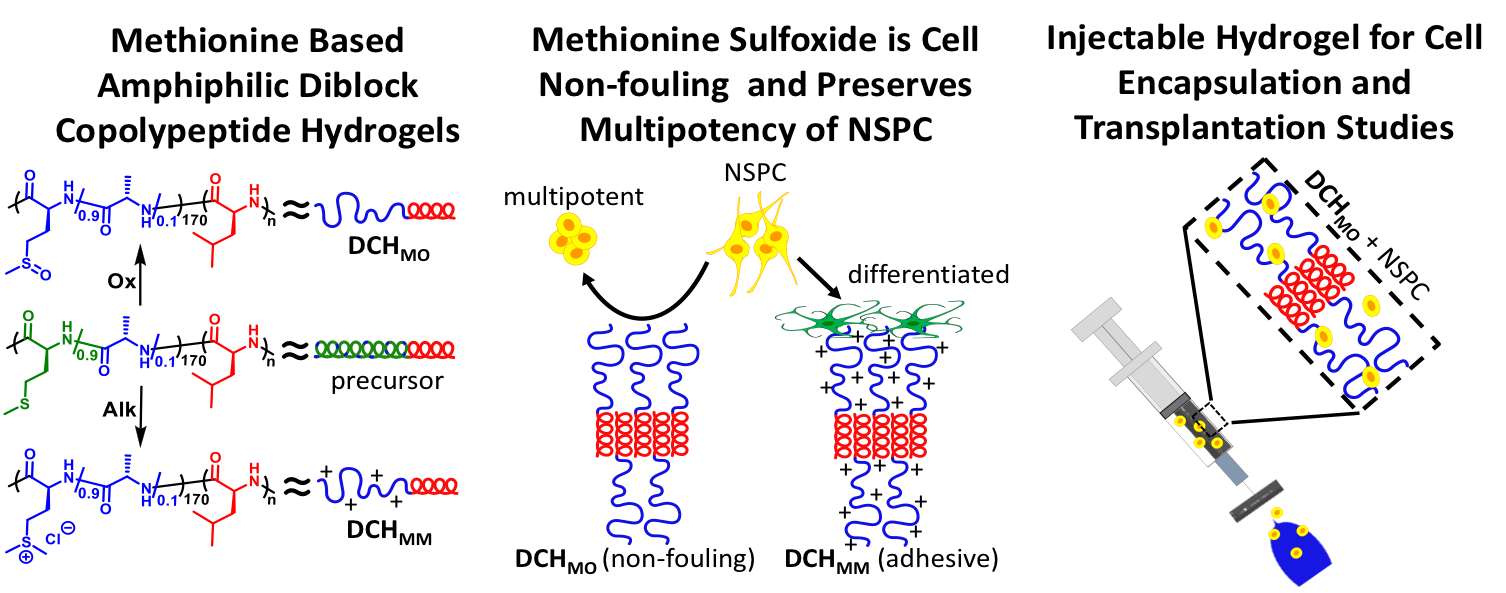
Poly(Methionine Sulfoxide) based Non-Ionic DCH
As
we continue to develop and optimize our DCH formulations, we have
switched from unnatural oligoethylene glycol based hydrophilic segments
in non-ionic DCH to use of natural poly(methionine sulfoxide) segments
(DCHMO). The sulfoxide functionality in these hydrogels
possesses non-fouling properties (similar to PEG), but gives DCH that
are degradable and resorbable, in addition to being less expensive and
easier to synthesize. DCHMO is currently being used for a variety of NPSC grafting studies in mice.
Wollenberg, A. L.; O’Shea, T. M.; Kim, J. H.; Czechanski, A.; Reinholdt, L. G.; Sofroniew, M. V.; Deming, T. J. Biomaterials, 2018, 178, 527-545.
Wollenberg, A. L.; O’Shea, T. M.; Kim, J. H.; Czechanski, A.; Reinholdt, L. G.; Sofroniew, M. V.; Deming, T. J. Biomaterials, 2018, 178, 527-545.
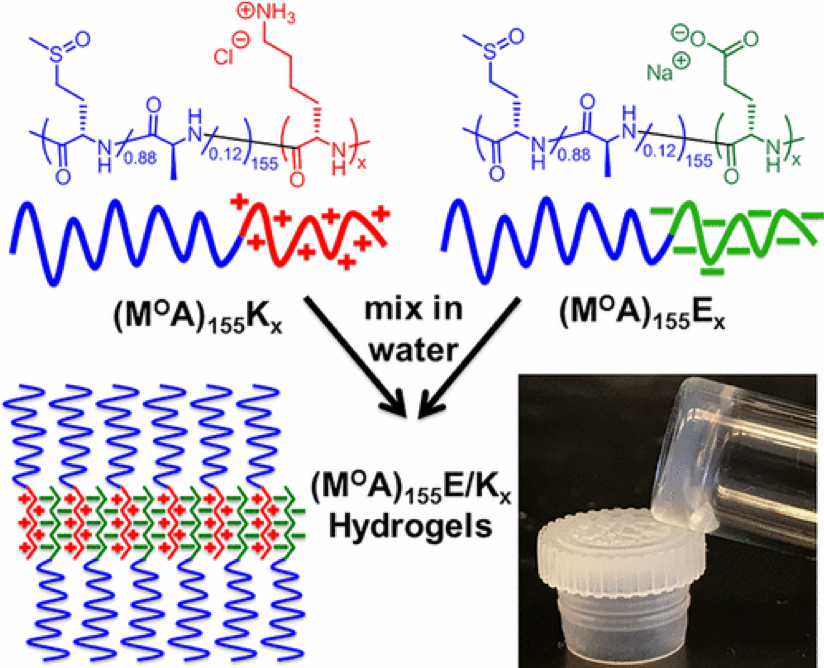
Polyion Complex DCH (DCHPIC)
Another
recent development has been the design of DCH that can assemble via
polyion complexation as opposed to hydrophobic interactions. By taking
advantage of the ability of enantiomerically pure poly(lysine) and
poly(glutamate) segments to form beta-sheet structured polyion
complexes, we were able to form DCH that retain the functionality of DCHMO, but are assembled via stronger electrostatic and H-bonding interactions. The result is DCHPIC that are significantly more resistant to dissolution compared to DCHMO, and can be prepared with much greater stiffness as two-component formulations while retaining cell and tissue compatibility.
Sun, Y.; Wollenberg, A. L.; O’Shea, T. M.; Cui, Y.; Zhou, Z. H.; Sofroniew, M. V.; Deming, T. J. J. Amer. Chem. Soc., 2017, 139, 15114–15121.
Sun, Y.; Wollenberg, A. L.; O’Shea, T. M.; Cui, Y.; Zhou, Z. H.; Sofroniew, M. V.; Deming, T. J. J. Amer. Chem. Soc., 2017, 139, 15114–15121.
 |
 |